Blog
Leveraging AI in GxP Environments: A Context-First Approach to Bioanalytical Data Management
In regulated bioanalytical laboratories, the promise of artificial intelligence has often seemed at odds with the stringent requirements of GxP compliance. Traditional approaches to AI implementation face significant hurdles: data privacy concerns, validation challenges, and the complexity of maintaining compliance when using external systems. Our solution takes a fundamentally different approach that maintains compliance while unlocking the power of AI for scientific data analysis.

The Context-First Paradigm
Rather than sending sensitive laboratory data to external AI systems – a practice that raises regulatory red flags – we’ve pioneered a context-first approach. Our solution leverages the advanced capabilities of existing Large Language Models (LLMs), which already excel at communication and information processing, and enhances them with specialized domain knowledge. By providing these LLMs with a comprehensive understanding of the data models used across different bioanalytical domains (LCMS, LBA, Immunogenicity), we enable intelligent analysis without exposing protected data.
This approach preserves the data within your validated environment while giving the AI the contextual understanding it needs to interpret scientific requests accurately. The AI becomes knowledgeable about your data structures, typical workflows, and domain-specific terminology without requiring access to the actual study data.
Leveraging LLMs with Domain-Specific Intelligence
Bioanalytical work encompasses distinct methodologies, each with unique data structures and analytical requirements:
- LCMS (Liquid Chromatography-Mass Spectrometry): Characterized by chromatograms, peak analysis, and calibration curves
- LBA (Ligand-Binding Assays): Focused on binding kinetics, parallelism, and dose-response relationships
- Immunogenicity: Centered on cutpoint determination, titer assessments, and confirmatory assays
By embedding domain knowledge directly into our AI system, we’re harnessing the inherent communication capabilities of modern LLMs while providing them with the specialized context they need to be truly useful in bioanalytical settings. These models are already remarkably effective at understanding natural language requests, and by augmenting them with domain-specific knowledge, we’ve created a solution that understands the nuances of each methodology. When a scientist requests an analysis, the AI comprehends not just the words but their specific meaning within the bioanalytical context.
Compliance by Design
This approach satisfies key GxP requirements:
- Data Integrity: Study data remains within your validated systems at all times.
- Traceability: All generated analyses include documented methodologies and validation steps.
- Reproducibility: Analysis modules create consistent results that can be regenerated as needed.
- Audit Trail: Every AI-assisted analysis maintains complete records of how it was created.
From Natural Language to Validated Analysis
This workflow demonstrates how powerful this approach can be:
1. A scientist is concerned on the device performance of his LCMS.
The app is already giving an analysis on “Retention Times”, but this is a too detailed analysis with plots of the retention times of all samples within a run for all runs of the study:
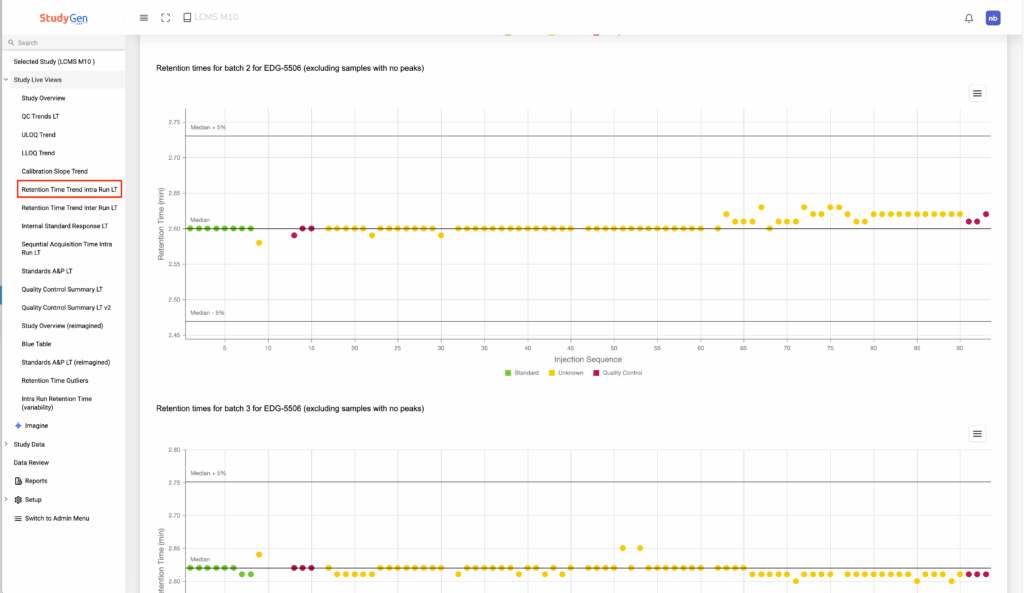
Based on this analysis, the scientist asks the app:
„Instead of charts, I want to see all data in a single table for all the runs. Only include the samples where the retention time is greater or less than 5% of the median for that run. Add the median and upper/lower limits for the run in the table too, and add the sample name.“
2. The context-aware AI interprets this request within the specific bioanalytical domain and the already defined analysis.
3. A validated analysis module is generated and shown:

4. The module can be integrated into your reporting platform using a defined IQ process.
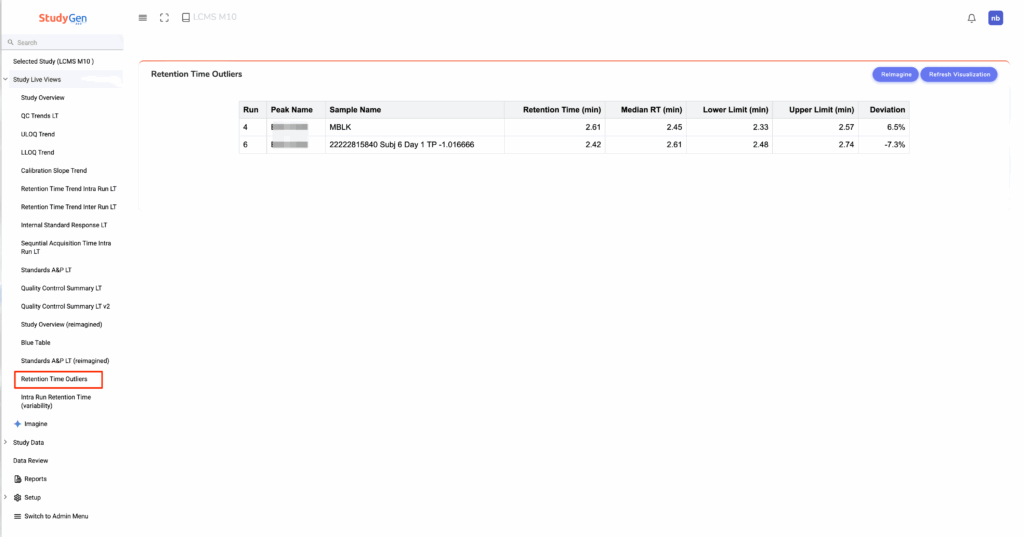
5. The module can be validated using the typical risk-based approach.
6. The analysis executes within your validated environment using your secured data
7. Results are presented with complete documentation of methods and parameters
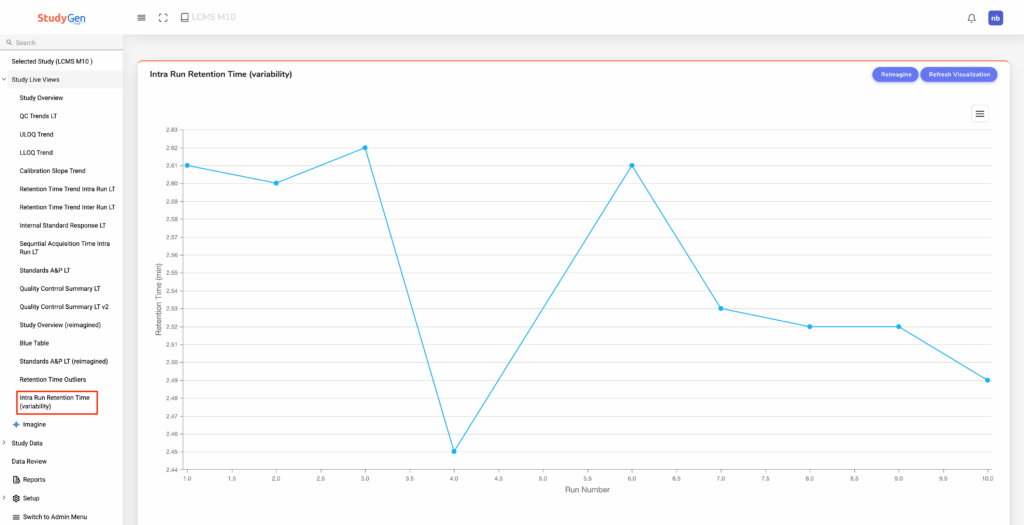
In the same way, by asking:
„Create a single chart with the median retention time vs the run number on the
x-axis.“
The scientist gets a plot that now shows the long-time behavior of the median retention time:
Real-World Benefits
This context-first AI approach delivers tangible advantages:
Future Directions
As regulatory frameworks evolve to address AI use in GxP environments, our context-first approach positions laboratories to adapt quickly. By keeping sensitive data within validated systems while leveraging AI for analytical intelligence, we’ve created a compliance-friendly path to innovation.
The future of bioanalytical laboratories lies not in sending data to AI, but in bringing AI intelligence to your data. Our solution demonstrates how existing LLM technology, when properly contextualized with domain knowledge, can transform scientific workflows while maintaining GxP compliance. This approach respects regulatory boundaries while empowering scientists with more intuitive and powerful analytical capabilities.
By focusing on practical AI implementation that solves real laboratory challenges, we’re helping bioanalytical teams embrace innovation without compromising the compliance foundation on which quality science depends.
This content might also be engaging for you!
-
CRO-Sponsor Collaboration: Digital Risk Mitigation
Blog Optimizing CRO-Sponsor Collaboration: Mitigating Risks in Bioanalytical Study Reporting Manual processes in bioanalytical studies jeopardise efficiency, data integrity,…
-
Context-First AI Approach for CROs | Multi-Run Assay Control
Blog The Critical Role of Multi-Run Assay Control in Bioanalytical Research: A Context-First AI Approach for Contract Research Organizations…
-
Standardized Multi-Run Assay Control Guide Recording
Blog Enabling Standardized Multi-Run Assay Control: Key Insights for Bioanalytical Labs In an increasingly complex regulatory environment, bioanalytical laboratories…